-
Shital Badhekar posted an update
Content Mannager at TIPNucleic Acid Therapeutics Market: Unleashing the Future of Precision Medicine
Introduction
The Nucleic Acid Therapeutics Market is experiencing a revolutionary boom, fueled by pioneering advances in RNA and DNA-targeted drug innovation. As precision medicine revolutionizes healthcare, nucleic acid therapies such as mRNA, siRNA, antisense oligonucleotides (ASOs), and gene-editing technologies are opening up new possibilities to cure once incurable or difficult-to-treat conditions. Ranging from rare genetic conditions to cancer and infectious diseases, this market is at the forefront of next-generation therapeutics.
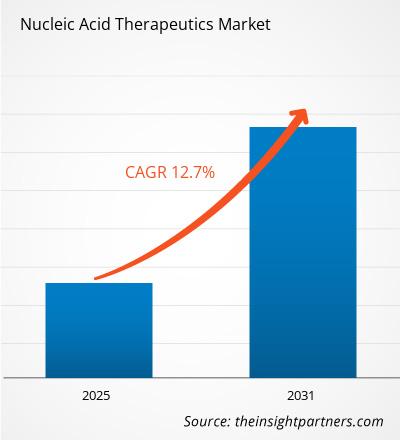
Nucleic Acid Therapeutics Market Overview
The Nucleic Acid Therapeutics Market is anticipated to have a CAGR of 12.7% during the period of 2025-2031 .Nucleic acid therapeutics employ artificial strands of nucleotides to either alter gene expression or repair defective genes. While traditional small molecule drugs or protein treatments attack the disease at the protein level, these attack the disease at the genetic level. Over the last couple of years, approvals of Spinraza (ASO), Onpattro (siRNA), and Comirnaty (mRNA vaccine) have established the commercial viability of this drug class.
Nucleic Acid Therapeutics Market Key Growth Drivers
•Growth in Genetic and Rare Diseases: Growing diagnosis of genetic diseases has established demand for focused, nucleic acid-based treatments.
•mRNA Vaccine Success: Success with COVID-19 mRNA vaccines has accelerated acceptance and development pipeline.
•Increased Investment and Partnerships: Biotech companies are drawing major investment, and big pharma players are partnering to amplify nucleic acid platforms.
• Advancements in Delivery Systems: Lipid nanoparticles (LNPs) and other new carriers are enhancing drug stability, bioavailability, and targeted delivery.
Nucleic Acid Therapeutics Market Key Applications
1. Infectious Diseases – mRNA vaccines for COVID-19, influenza, RSV.
2. Genetic Disorders – ASOs and siRNAs for rare diseases such as spinal muscular atrophy, Duchenne muscular dystrophy.
3. Oncology – Nucleic acid-based immunotherapies and customized cancer vaccines.
4. Cardiovascular & Metabolic Diseases – Gene silencing to modulate cholesterol and blood glucose levels.
Sample PDF Guide: – https://www.theinsightpartners.com/sample/TIPRE00027648
Recent Breakthroughs
•CRISPR-Cas Gene Editing: Intellia and CRISPR Therapeutics are leading in vivo editing technologies.
•Self-Amplifying RNA (saRNA): Next-generation mRNA tech providing longer expression at lower doses.
•Circular RNA (circRNA): Showing up as a more stable linear RNA alternative, with increasing R&D attention.
Nucleic Acid Therapeutics Market Key Players and their recent innovations
Alnylam Pharmaceuticals, Inc.
•FDA approval of Amvuttra® (vutrisiran): FDA approved Amvuttra in March 2025 for treating transthyretin-mediated amyloidosis with cardiomyopathy, the first RNAi therapy demonstrated to decrease cardiovascular mortality, hospitalizations, and urgent heart failure visits.
•Next-gen delivery platforms: Alnylam is also developing tissue-targeted RNAi delivery systems for adipose, muscle, heart, and kidney with a goal to move beyond the liver with RNAi accessibility by 2030.
Silence Therapeutics plc
•Polycythemia Vera Phase 1 Divesiran data: Their mRNAi GOLD platform achieved encouraging Phase 1 data for Divesiran, a first-in-class siRNA therapeutic for polycythemia vera.
•Zerlasiran Phase 2 results: Shown to achieve sustained decreases in lipoprotein(a) levels at 60 weeks, supporting its cardiovascular pipeline.
Arbutus Biopharma Corporation
•Reacquisition of China rights on Imdusiran (AB-729): Now advancing development of this anti-HBV siRNA candidate under an original Sanofi agreement.
• Liver-targeting siRNA patent: Acquired IP for new double-stranded siRNA conjugates for HBV/HBV treatment.
Genzyme (Sanofi)
• RNA platform investment: Sanofi (Genzyme) is developing internal expertise in mRNA therapeutics and vaccines through AI-powered biologic development
Ionis Pharmaceuticals
• FDA approval of Tryngolza® (olezarsen): Ionis introduced in December 2024 its first wholly owned medicine for familial chylomicronemia syndrome, lowering triglycerides and risk of pancreatitis.
•NDA approval for Donidalorsen: Gained FDA priority review for prophylactic treatment of hereditary angioedema.
Sarepta Therapeutics
•Strategic shift to siRNA assets: After restructuring mid-2025, Sarepta is focusing on siRNA platforms over gene therapy, in spite of troubles with Elevidys.
•Ongoing focus on Elevidys: In spite of label caution and layoffs, the gene therapy is being reassessed with safety measures, given protein expression data remains robust (~94%).
Benitec Biopharma Inc.
•BB-301 siRNA therapy advances: Encouraging interim data were announced for BB 301 in a Phase 1b/2a trial for muscular dystrophy.
•ETERS towards combined gene-silencing therapy: Benitec is developing a hybrid gene therapy + RNAi platform (ddRNAi) aimed at revolutionizing treatment paradigms.
Arrowhead Pharmaceuticals
•TRiM™ platform expansion: The TRiM RNAi discovery development platform continues to drive effective discovery and candidate progression.
• Plozasiran Phase 3 completion: Trials enrollment completed in severe hypertriglyceridemia disorders, progressing toward partner and independent commercialization in 2025.
Biogen, Inc.
• Recent news did not indicate any significant new nucleic acid therapeutic innovation in the past year as of mid 2025. Biogen still has ongoing broader pipelines but without clear public milestones in RNA-based drugs.
Nucleic Acid Therapeutics Market Challenges Ahead
•Delivery Challenges: Effective and targeted delivery to tissues such as brain and muscle is still an obstacle.
•Regulatory and Manufacturing Complexity: Therapeutic nucleic acids demand special approval pathways and manufacturing facilities.
•Long-Term Safety: Since most of these treatments are novel, long-term safety and efficacy are still under thorough post-market monitoring.
Nucleic Acid Therapeutics Market Outlook
The market for global nucleic acid therapeutics is anticipated to surpass USD 30 billion in 2030, advancing at a CAGR of more than 15% over the forecast period. Driven by a lucrative pipeline of products, partnerships, and growing patient awareness, the industry is poised to become a cornerstone of personalized medicine.
Conclusion
The market for nucleic acid therapeutics is at the cutting edge of medical science. By leveraging the genetic code of life, it promises accurate, targeted, and perhaps curative care. As understanding, regulation, and delivery science advance, medicine’s future is being written in nucleic acid terms more and more every day.
Nucleic Acid Therapeutics Market Frequently Asked Questions (FAQs)
Q1. What are nucleic acid therapeutics?
A1. Nucleic acid medicines are pharmaceuticals constructed from DNA or RNA that regulate gene expression to cure illnesses at the molecular level.
Q2. How do mRNA, siRNA, and ASO differ from each other?
A2. mRNA provides instructions for protein synthesis; siRNA and ASO silence or inhibit gene expression to halt the generation of aberrant proteins.
Q3. Are nucleic acid treatments safe?
A3. All approved therapies have shown good safety profiles, although long-term safety continues to be followed, particularly for new technologies.
Q4. What diseases can be treated with nucleic acid drugs?
A4. The therapies are applied to genetic disease, cancer, infectious diseases, and new applications in cardiovascular and neurological diseases.
Q5. What are the main delivery methods?
A5. Lipid nanoparticles (LNPs), conjugates (such as GalNAc for liver targeting), and viral vectors are the most frequent delivery systems.
theinsightpartners.com
Nucleic Acid Therapeutics Market Forecast (2025-2031)
Nucleic Acid Therapeutics Market to achieve a CAGR of 12.7% by 2031. Understand the complex interplay of influential factors including drivers, challenges, and opportunities